Answers
Answer:
alkali metals is your answer
Answer:
alkali metals
Explanation:
Related Questions
Solder is a silver metal used to hold pipes together. When the solder is heated, it melts and acts as a type of metal "glue." Mrs. Hanley heats a piece of solder until it melts between two pipes. What best identifies the point at which a physical change first takes place?
A)when the solder melts
B)when the solder cools down
C)when the solder is a soft metal
D)when the solder becomes solid again
Answers
Answer:
A
Explanation:
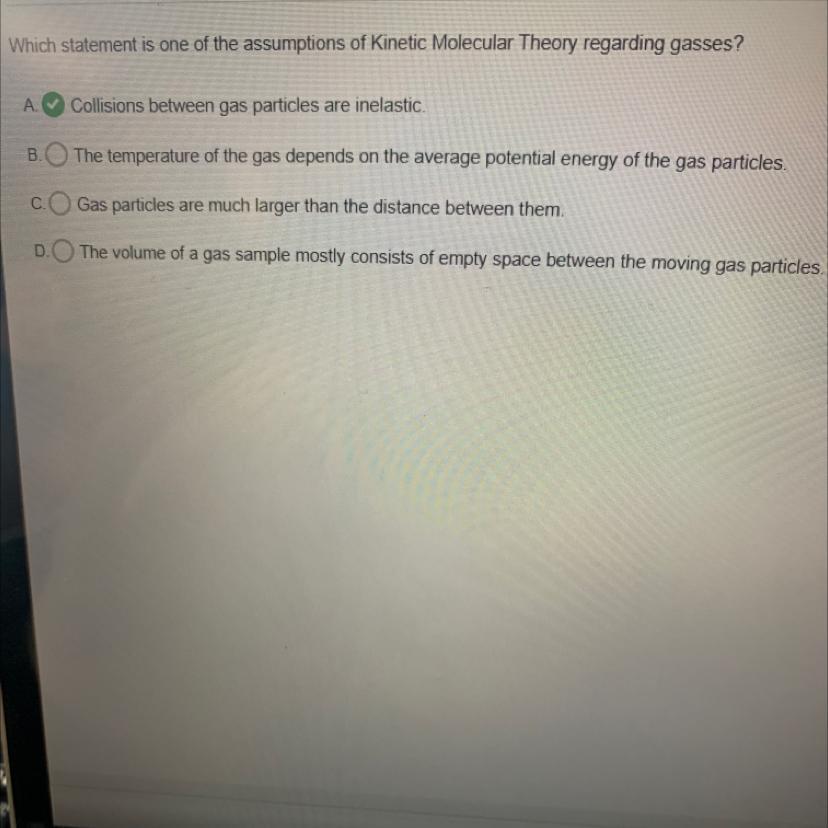
Which statement is one of the assumptions of Kinetic Molecular Theory regarding gasses?
A Collisions between gas particles are inelastic.
The temperature of the gas depends on the average potential energy of the gas particles.
Gas particles are much larger than the distance between them.
The volume of a gas sample mostly consists of empty space between the moving gas particles.
Answers
The statement that "The volume of a gas sample mostly consists of empty space between the moving gas particles" is one of the assumptions of the Kinetic Molecular Theory regarding gases.
What is Collision?
There are different types of collisions, depending on the nature of the objects involved, the speed and direction of their motion, and the type of contact that occurs. For example, elastic collisions are those in which the total kinetic energy of the colliding objects is conserved, meaning that no energy is lost or gained during the collision. In contrast, inelastic collisions are those in which some of the kinetic energy is transformed into other forms of energy, such as heat or sound.
The Kinetic Molecular Theory is a model that describes the behavior of gases. One of the main assumptions of this theory is that gas particles are in constant random motion and move in a straight line until they collide with other particles or the walls of their container.
Another important assumption of this theory is that the volume of a gas sample mostly consists of empty space between the moving gas particles. This means that gas particles are assumed to be very small compared to the overall volume of the gas sample. Therefore, the particles do not occupy all of the available space in the container, but instead only occupy a small portion of it.
Learn more about Collision from given link
https://brainly.com/question/24915434
#SPJ1
When an object floats in water, it displaces a volume of water that has a weight equal to the weight of the object. If a ship has a weight of 4247 tons, how many cubic feet of seawater will it displace? Seawater has a density of 1.025 g·cm-3; 1 ton = 2000 lb. (Enter your answer in scientific notation.)

Answers
The amount of seawater that will be displaced would be 1.3274 x \(10^5\) \(ft^3\)
Density and volumeDensity = mass/volume.
Since a floating object displaces its own weight, a 4247 tons ship will also displace the same amount of water.
4247 tons of seawater = 3.8528 x \(10^9\) g
volume of 3.8528 x \(10^9\) g seawater = mass/density = 3.8528 x \(10^9\) g/1.025 = 3758829268.29 cubic centimeter.
3758829268.29 \(cm^3\) = 132741.80 \(ft^3\) or 1.3274 x \(10^5\) \(ft^3\)
More on density can be found here: https://brainly.com/question/15164682
#SPJ1
What is the wavelength of a 229 Hz sound wave moving at a speed of 345 m/s?
Answers
Answer:
wave length=speed/frequency
=345/229
27. The unit of volume in the metric system is the liter,
which consists of 1000 milliliters. How many liters or
milliliters is each of the following common English
system measurements approximately equivalent to?
a. a gallon of gasoline
b. a pint of milk
c. a cup of water
Answers
Liters and millilitres are the most common units of volume.
The volume equal to a gallon of gasoline is 3785 m L which is equal to 3.785 L.
The volume equal to pint of milk is 473...
A a cup of water is 0.24 L
which of the following is best described by the equation H2o (I) to H2O (s) + heat
Answers
Exothermic means that the temperature rises. The following equation represents the freezing of water to make ice. H2O(l) → H2O (s) Exothermic; energy is generated as water molecules form bonds during the phase transition.
Which phase transition is an exothermic formula?An exothermic phase transition occurs when the sample loses energy (heat) to the environment. Condensation is the right answer since it is the phase transition from gas to liquid.
Condensation is the phase shift that occurs when a material transitions from a gas or vapour to a liquid. The process of condensation is exothermic.
When the energy created in an exothermic reaction is released as heat, the temperature rises.
Learn more about exothermic phase
https://brainly.com/question/1875234
#SPJ1
An experimental measurement was taken of 10.4mL and the actual measurement was 9.7mL. What is the percent error?
Answers
Answer:
13%
Explanation:
Can the melting point of a substance be used as its fingerprint?
Answers
Answer:
Melting and boiling points are unique to the chemicals just like facial recognition. That does not necessarily means it is like a fingerprints.
Explanation:
please rate as brainliest
How much potassium chloride will dissolve in 25 grams of water at 80°C?
Please I need help
Answers
Answer:
The problem provides you with the solubility of potassium chloride,
KCl
, in water at
20
∘
C
, which is said to be equal to
34 g / 100 g H
2
O
.
This means that at
20
∘
C
, a saturated solution of potassium chloride will contain
34 g
of dissolved salt for every
100 g
of water.
As you know, a saturated solution is a solution that holds the maximum amount of dissolved salt. Adding more solid to a saturated solution will cause the solid to remain undissolved.
In your case, you can create a saturated solution of potassium chloride by dissolving
34 g
of salt in
100 g
of water at
20
∘
C
.
Now, your goal here is to figure out how much potassium chloride can be dissolved in
300 g
of water at this temperature. To do that, use the given solubility as a conversion factor to take you from grams of salt to grams of water
which of the following samples will have the greatest volume at stp? a) 22 g co b) 22 g he c) 22 g o2 d) 22 g cl2 e) all of these samples would have the same volume at stp.
A) 22 g He B) 22 g Cl2 C) 22 g CO D) 22 g 02 E) All of these samples would have the same volume at STP.
Answers
The answer is (e) all of the samples have same volumes at STP.
What is STP?
At standard temperature and pressure (STP), all gases have the same volume, regardless of their mass or chemical composition. STP is defined as a temperature of 273 K (0°C) and a pressure of 1 atm.
At STP, all gases have a density of approximately 1.225 kg/m3, and the volume of a gas is directly proportional to its number of moles. Therefore, any sample of a gas at STP will have the same volume as any other sample of the same number of moles.
To determine the number of moles in a sample of a gas, you can use the ideal gas law, which states that the volume of a gas is directly proportional to the number of moles of the gas and inversely proportional to the pressure and temperature:
PV = nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature of the gas.
If you know the mass and molecular weight of the gas, you can also use the following equation to calculate the number of moles:
n = (m) / (MW)
where m is the mass of the gas, and MW is the molecular weight of the gas.
Therefore, to determine which of the given samples would have the greatest volume at STP, you would need to calculate the number of moles in each sample and compare them. All of the samples would have the same volume if they had the same number of moles.
Hence, all of the samples have the same volume at STP.
To learn more about standard temperature and pressure from the given link:
https://brainly.com/question/14820864
#SPJ4
Can you guys help me answer this ?



Answers
Answer:
the picture is kinda blurry
Explanation:
Which characteristic is expected of a mineral that crystallizes from lava at Earth’s surface?
Answers
Answer:
Large Crystal
Explanation:
Yosoro!✨✨✨
Predict whether aqueous solutions of the following substances are acidic, basic, or neutral:
(a) AlCl3
(b) NaBr
(c) NaClO
(d) 3CH3NH34NO3
(e) Na2SO3
Answers
Answer:
A. Acidic
B. Neutral
C. Basic
D. Acidic
E. Basic
Explanation:
The reaction of salts with water to give aqueous solutions which are either acidic, basic or neutral is known as salt hydrolysis. Salts that are produced from the reaction of strong acids and weak bases give acidic solutions. Salts that are produced the reaction between weak acids and strong bases give basic solutions. Neutral solutions are obtained from salts produced bynthe reaction of strong acids and strong bases.
The hydrolysis of the given salts are shown below:
A. AlCl₃ + 3 H₂O ---> 3 HCl + Al(OH)₃
The aqueous solution produced will be acidic since HCl is a strong acid while Al(OH)₃ is a weak base.
B. NaBr + H₂O ----> HBr + NaOH
The aqueous solution produced will be neutral since HBr is a strong acid and NaOH is strong base as well.
C. NaClO + H₂O ----> HClO + NaOH
The aqueous solution produced will be basic since HClO is a weak acid while NaOH is a strong base.
D. CH₃NH₃NO₃ + H₂O ----> HNO₃ + CH₃NH₂ + H₂O
The aqueous solution produced will be acidic since HNO₃ is a strong acid while CH₃NH₂ is a weak base.
E. Na₂SO₃ + 2 H₂O ----> H₂SO₃ + 2 NaOH
The aqueous solution produced will be basic since H₂SO₃ is a weak acid while NaOH is a strong base.
Based on the products formed when they react with water, the following is true:
AlCl₃ - Acidic. NaBr - Neutral.NaClO - Acidic.3CH₃NH₃NO₃ - Acidic.Na₂SO₃ - Basic.What happens when the above compounds react with water?When AlCl₃ reacts with water, it produces Hydrochloric acid which is a strong acid. The solution will be acidic.
NaBr reacting with water produces a strong base in Sodium Hydroxide and a strong acid in Hydrobromic acid. As they are both strong, they will cancel the other out and leave a neutral solution.
NaClO will produce a weak acid and a strong base which is Sodium Hydroxide. Solution will be basic.
3CH₃NH₃NO₃ will produce a weak base and a strong acid - Nitric Acid - which would make the solution acidic.
Na₂SO₃ would produce the strong base Sodium Hydroxide and a weak acid so the solution will be basic.
Find out more on bases and acids at https://brainly.com/question/15486690.
what volume litters of oxygen would be ptoduced in the electrolysis which forms 548 litters of hydrogen both gases measured at stp?
Answers
The ideal gas law may be used to determine the volume of oxygen created in the electrolysis that produces 548 litres of hydrogen at STP (Standard Temperature and Pressure). PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature, according to the ideal gas equation.
The pressure is 1 atm, the temperature is 273 K, and the number of moles of hydrogen is 548/22.4 = 24.5 in this example. We may compute the volume of oxygen created by rearranging the ideal gas law: V = nRT/P = 24.5*0.082*273/1 = 483.3 litres.
As a result, the volume of oxygen created in the electrolysis at STP that produces 548 litres of hydrogen is 483.3 litres.
Learn more about oxygen at:
https://brainly.com/question/2272415
#SPJ1
Someone help me do each piece of evidence in 2-4 sentences
No definitions
No go*gle
Claim 1: The Moon’s appearance changes because Earth casts a shadow on the Moon.
Claim 2: The Moon’s appearance changes because the position of the Moon changes.
Answers
Claim 1: occurs during full moon phase
Claim 2: the orbital function of the moon to cast full moon, new moon and crescent moon phases.
How are the moon appearances described?Claim 1: The Moon's appearance changes due to Earth casting a shadow on it during a lunar eclipse. During this event, the Earth is positioned between the Moon and the Sun, and the Earth's shadow falls on the Moon, creating a reddish-brown hue. This phenomenon can only occur during a full moon phase.
Claim 2: The Moon's appearance changes due to its position relative to the Earth and the Sun. As the Moon orbits around the Earth, the amount of sunlight that reflects off of it changes, causing different phases, such as full moon, new moon, and crescent moon. Additionally, the Moon's position in the sky changes over time, which can affect its visibility and apparent size.
Find out more on moon appearance here: https://brainly.com/question/20711283
#SPJ1
Balance the following chemical equation:
___HCO + ___O —> ___H2 + ___CO3
Answers
Answer:
Balanced Equation:
2HCO + 4O –––> H2 + 2CO3
A very simple assumption for the specific heat of a crystalline solid is that each vibrational mode of the solid acts independently and is fully excited and thus cv=3NAkB=24.9 kJ/(kmol⋅K). This is called the law of Dulong and Petit. Calculate the Debye specific heat (in units of kJ/(kmol⋅K) of diamond at room temperature, 298 K. Use a Debye temperature of 2219 K.
Answers
Answer:
4.7 kJ/kmol-K
Explanation:
Using the Debye model the specific heat capacity in kJ/kmol-K
c = 12π⁴Nk(T/θ)³/5
where N = avogadro's number = 6.02 × 10²³ mol⁻¹, k = 1.38 × 10⁻²³ JK⁻¹, T = room temperature = 298 K and θ = Debye temperature = 2219 K
Substituting these values into c we have
c = 12π⁴Nk(T/θ)³/5
= 12π⁴(6.02 × 10²³ mol⁻¹)(1.38 × 10⁻²³ JK⁻¹)(298 K/2219 K)³/5
= 9710.83(298 K/2219 K)³/5
= 1942.17(0.1343)³
= 4.704 J/mol-K
= 4.704 × 10⁻³ kJ/10⁻³ kmol-K
= 4.704 kJ/kmol-K
≅ 4.7 kJ/kmol-K
So, the specific heat of diamond in kJ/kmol-K is 4.7 kJ/kmol-K
Explain why the following picture illustrates the relationships between voltage, current and resistance. (hint: start with Ohm's Law)

Answers
Answer:
Explanation:
We can see that this picture can be used to show Ohm's Law graphically so it illustrates Ohm's Law.
According to Ohm's Law electric current is directly proportional to voltage and inversely proportional to resistance.
Mathematically, V ∝ I,
or V=IR,
where, V ⇒ voltage difference between two points,
I ⇒ current flowing through the resistance,
R ⇒ proportionality constant or resistance.
According to the picture voltage ( SI unit Volt ) is supporting the current ( SI unit Ampier ) to move out from the barrier while the resistor ( SI unit Ohm )is acting as a barrier to its way . As it is satisfying the Ohm's Law it illustrates the relationship between voltage, current, and resistance.
A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one compartment, occupying one-third of the total volume, contains air at 500oR, and the other is evacuated. The valve is opened and the air is allowed to fill the entire volume. Assuming the ideal gas model with variable specific heats. Determine: a. the final temperature of the air (in oR) b. the amount of specific entropy produced (in Btu/lbm oR)
Answers
Answer:
a) the final temperature of the air is 500° R
b) the amount of specific entropy produced is 0.0758 Btu/lb-°R
Explanation:
Given the data in the question;
Air at 500° R = \(T_i\)
Using first law of thermodynamic;
δQ = dU + W
now, since the vessel is insulated, the transfer is zero, work done also is zero since there is also no external work done.
δQ = dU + W
0 = dU + 0
dU = 0
\(u_f\) - \(u_i\) = 0
\(u_f\) = \(u_i\)
hence, change in internal energy is 0
Now, since the ideal internal energy is a function of temperature, the temperature will also remain the same;
\(T_f = T_i\)
F = 500° R
Therefore, the final temperature of the air is 500° R
b)
given that; initial volume is one-third of the total volume
V₁ = \(\frac{1}{3}\)V₂
3V₁ = V₂
3 = V₂/V₁
Now, we take the value of gas constant R from air property table; gas constant R = 0.069 Btu/lb-R
so we calculate the entropy change;
Δs = \(c_v\)In( \(\frac{T_2}{T_1}\) ) + R.In( \(\frac{V_2}{V_1}\) )
we substitute
Δs = \(c_v\)In( \(\frac{500}{500}\) ) + 0.069 × In( 3 )
Δs = 0 + [0.069 × In( 3 )]
Δs = 0 + [0.069 × 1.0986]
Δs = 0.0758 Btu/lb-°R
Therefore, the amount of specific entropy produced is 0.0758 Btu/lb-°R
Insulated vessels separate the environment of the outer and the inner system. The final temperature is 500 degrees R and 0.0758 Btu/lb- degree R is the entropy.
What is temperature?The temperature is the measure of the hot or the coldness of the system. The first law of the thermodynamics is used to measure the final temperature of the system:
\(\rm \Delta Q = \rm \Delta U + W\)
The work done will be zero as the system is insulated and no external work is being done.
\(\begin{aligned} \rm 0 &= \rm \Delta U + 0\\\\\rm U_{f} - U_{i} &= 0\\\\\rm U_{f} &= \rm U_{i} \end{aligned}\)
Hence, the change in the internal energy is zero. Thus, the final temperature will remain the same,
\(\rm T_{f} = \rm T_{i} = 500 ^{\circ} \rm R\)
Now, as we know, the initial volume is one-third of the total volume then,
\(\begin{aligned} \rm V_{1} &= \rm \dfrac{1}{3} V_{2}\\\\\rm 3V_{1}&= \rm V_{2}\\\\3 &= \rm \dfrac{V_{1}}{V_{2}}\end{aligned}\)
The change in entropy is calculated as:
\(\begin{aligned} \rm \Delta S &= \rm C_{v} ln ( \dfrac{T_{2}}{T_{1}}) + R \times ln ( \dfrac{V_{2}}{V_{1}}) \\\\&= 0 + [0.069 \times \rm ln( 3 )]\\\\& = 0.0758 \;\rm Btu/lb-^{\circ}R \end{aligned}\)
Therefore, the entropy produced is 0.0758 Btu/lb- degree R.
Learn more about entropy here:
https://brainly.com/question/19538748
For this assignment, you will make a scale model—a “core sample”—showing the layers of the Earth: crust, lithosphere, asthenosphere, mantle, outer core, and inner core.
Answers
-A large, clear plastic container
-A ruler
-A variety of different colored modeling clay
-A sharp knife
-A marker
Instructions:
1. Measure the height of the plastic container and mark it with the marker.
2. Cut the modeling clay into thin slices with the knife.
3. Begin layering the clay slices in the container, starting with the crust at the bottom.
4. Layer the lithosphere, asthenosphere, mantle, outer core, and inner core in order.
5. Use the ruler to measure the thickness of each layer and mark it with the marker.
6. Once all the layers are in place, your scale model of the Earth's layers is complete!
Hope that helps !?
Cardboard or foam board
Scissors
Glue
Markers or paint
Ruler
First, decide on a scale for your model. For example, you could use a scale of 1 inch = 1000 miles. This means that for every 1 inch on your model, it represents 1000 miles in the actual Earth.
Next, use your ruler to measure and cut out strips of cardboard or foam board to represent the layers of the Earth. The crust is the outermost layer and is relatively thin, while the inner core is the innermost layer and is the thickest. Use your markers or paint to label each layer and add any additional details or information you would like to include on your model.
Finally, glue the layers together in the correct order, starting with the crust at the top and ending with the inner core at the bottom. Your scale model of the Earth's layers is now complete!
Translation is the conversion of
1 .RNA to DNA.
2. DNA to RNA.
3. DNA to protein.
4. RNA to protein.
(WILL MARK BRAINLIEST)
Answers
Translation is the process of translation messenger RNA to a chain of amino acids.
Translation is the conversion of RNA to protein. The conversion of RNA to DNA is called reverse transcription.
What do you mean by Proteins?Proteins may be defined as natural-appearing, extremely complex substances that consist of amino acid residues joined by peptide bonds.
The process of translation occurs in the cytoplasm of the cells, while the process of transcription occurs in the nucleus itself.
The conversion of DNA to RNA is called transcription. While the proteins are never directly converted from DNA, it involves both processes of transcription followed by the translation.
Therefore, translation is the conversion of RNA to protein.
To learn more about Translation, refer to the link:
https://brainly.com/question/2449073
#SPJ2
What are the characteristics of chemical reaction?
Answers
Hope this answer helps you

20. What is an irreversible change?
Answers
Answer:
A change is called irreversible if it cannot be changed back again. For example you cannot change a cake back into its ingredients again. Irreversible changes are permanent.
Explanation:
Hope this helps!! :))
Many people have said that cold water boils faster than hot water. This is not true. In fact, it’s been said so many times that most people believe it to be a fact. Postulate a reason for why this may have been thought to be true. Is there any scientific evidence backing this claim at all? Please explain your reasoning.
Answers
The claim that cold water boils faster than hot water is not true. The reason why this misconception may have emerged is likely due to a misunderstanding or misinterpretation of certain observations. However, there is no scientific evidence supporting this claim.
One possible reason for this misconception is the notion that hot water takes longer to reach its boiling point because it starts at a higher temperature. When comparing hot and cold water in terms of reaching the boiling point from room temperature, the cold water may appear to boil faster.
However, this is simply because the hot water has already gained a head start in terms of temperature. In reality, once both liquids reach their respective boiling points, the hot water will boil first.
Scientifically, the boiling point of water is determined by its temperature and pressure. Under normal atmospheric conditions, the boiling point of water is 100 degrees Celsius (212 degrees Fahrenheit). Heating water raises its temperature, and once it reaches 100 degrees Celsius, it transitions into the gaseous state. The initial temperature of the water does not affect the boiling point itself.
In conclusion, the claim that cold water boils faster than hot water is a misconception. It likely arose from a misinterpretation of observations, and there is no scientific evidence to support this claim. The boiling point of water is solely determined by its temperature and pressure, regardless of whether the water is initially hot or cold.
for such more questions on evidence
https://brainly.com/question/6273210
#SPJ8
For the chemical reaction:
N2 (g)
+
H2(g)=
NH3 (g)
Calculate the volume of NH; that will form from 200 dm cubed of N2 at STP. (N = 14; H - 1)
[At STP. 1 mole of any gas occupies volume of 22 A dm cubed
Answers
Answer:
Volume of ammonia produced = 398.7 dm³
Explanation:
Given data:
Volume of N₂ = 200 dm³
Pressure and temperature = standard
Volume of ammonia produced = ?
Solution:
Chemical equation:
N₂ + 3H₂ → 2NH₃
Number of moles of N₂:
PV = nRT
1 atm× 200 L = n× 0.0821 atm.L/mol.K × 273 K
n = 200 atm.L /22.41 atm.L/mol
n = 8.9 mol
Now we will compare the moles of ammonia and nitrogen.
N₂ : NH₃
1 : 2
8.9 : 2/1×8.9 = 17.8 mol
Volume of ammonia:
1 mole of any gas occupy 22.4 dm³ volume
17.8 mol ×22.4 dm³/1 mol = 398.7 dm³
A gas occupying 50.0 ml volume in a confined space at 20.0 dc at 50.0 kpa is heated to 40.0 dc. What is the pressure exerted by the gas in the container?
Answers
Answer:The pressure exerted by the gas is 100kPa
Explanation:Let's apply the Charles Gay Lussac law, to solve the question.
At constant volume, the pressure varies proportionally with the temperature.
P initial / T° initial = P final / T° final
50kPa / 20°C = P final / 40°C
Temperature has increased the double, so the pressure will be increased, the double too.
100 kPa
If you need more help go to this link https://brainly.com/question/14378507
Two students are engaged in an argument about whether or not a plant can live independently inside of a sealed jar for up to two weeks by using energy from the sun and energy from the mitochondria to carry out all of its life processes.
Student A: The plant can live independent of other organisms such as animals, because it can obtain nutrients to grow, reproduce, make needed materials, and remove the waste on its own.
Student B: The plant only relies on other organisms, such as animals that produce the carbon dioxide needed to obtain nutrients to grow, reproduce, make needed materials, and remove waste.
Based on the diagram, which student's argument is BEST supported? Use evidence from the diagram to support your reasoning.
Answers
Answer:
where's the diagram?
Explanation:
the best answer is student A
Which emission spectrum represents the copper?
A.) Spectrum A
B.) Spectrum B
C.) Spectrum C

Answers
Answer:b
Explanation:i just did it
Which physical method can be used for obtaining a sample of salt from a small beaker of salt water?
boiling
freezing
chromatography
sorting
Answers
Answer:
a. boiling
Explanation:
Phosphorous trichloride (PCl3) is produced
from the reaction of white phosphorous (P4)
and chlorine:
P4(s) + 6 Cl2(g) → 4 PCl3(g).
A sample of PCl3 of mass 262.6 g was collected
from the reaction of 79.12 g of P4 with excess
chlorine. What is the percentage yield of the
reaction?
Answer in units of %.
Answers
The percentage yield obtained from the given reaction above is 74.8%
Balanced equationP₄ + 6Cl₂ → 4PCl₃
Molar mass of P₄ = 31 × 4 = 124 g/mol
Mass of P₄ from the balanced equation = 1 × 124 = 124 g
Molar mass of PCl₃ = 31 + (35.5×3) = 137.5 g/mol
Mass of PCl₃ from the balanced equation = 4 × 137.5 = 550 g
SUMMARYFrom the balanced equation above,
124 g of P₄ reacted to produce 550 g of PCl₃
How to determine the theoretical yieldFrom the balanced equation above,
124 g of P₄ reacted to produce 550 g of PCl₃
Therefore,
79.12 g of P₄ will react to produce = (79.12 × 550) / 124 = 350.9 g of PCl₃
How to determine the percentage yield Actual yield of PCl₃ = 262.6 gTheoretical yield of PCl₃ = 350.9 gPercentage yield =?
Percentage yield = (Actual /Theoretical) × 100
Percentage yield = (262.6 / 350.9) × 100
Percentage yield = 74.8%
Learn more about stoichiometry:
https://brainly.com/question/14735801