When would we choose to use a burner in the organic lab?
Answers
A burner is an essential tool in organic chemistry labs as it is used for various purposes such as heating, sterilization, and combustion of organic materials. The choice to use a burner depends on the specific experiment or procedure being carried out.
For example, a burner may be used to heat a reaction mixture to initiate a chemical reaction or to evaporate solvent from a solution.
In addition, a burner may be used to sterilize equipment and glassware to prevent contamination of the experiment. This is particularly important when working with microorganisms or sensitive biological samples. Burners can also be used to combust organic materials such as solid waste or excess reactants.
Overall, the use of a burner in the organic lab depends on the specific experiment and the requirements of the procedure. However, it is important to remember that safety measures must always be followed when using a burner, such as proper ventilation, appropriate clothing, and handling of the equipment with care.
learn more about burner here
https://brainly.com/question/10281181
#SPJ11
Related Questions
Fill in the chart with the following words:
- Potential energy
- kinetic energy
- Chemical energy
- Joules
- Energy
- thermal energy

Answers
Answer:
Energy
potential energy kinetic energy
chemical energy thermal energy
joules
Explanation:
chemical energy is a type of potential energy or stored energy. Heat is a kinetic energy and energy is measured in joules.
The first box is energy. It can be divided into kinetic energy in one side and potential energy in the other side. Here, kinetic energy can be converted to the thermal energy and potential energy can be converted to chemical energy. They are measure in Joules.
What are various forms of energy ?Potential energy and kinetic energy are two forms of energy that are commonly encountered in physics.
Potential energy is energy that an object possesses due to its position, shape, or state. An object can store potential energy when it is moved away from its equilibrium position, such as when a spring is stretched or a book is lifted to a certain height above the ground.
The amount of potential energy an object has is proportional to its mass, height, and the strength of the force that is acting on it. The average kinetic energy of an object is called its thermal energy.
Therefore, first box is energy. It can be divided into kinetic energy in one side and potential energy in the other side. Here, kinetic energy can be converted to the thermal energy and potential energy can be converted to chemical energy. They are measure in Joules.
Find more on potential energy:
https://brainly.com/question/11749818
#SPJ2
99.9% of the air particles are found in the lower 50 kilometres of the atmosphere. What does this say about the density of the air near Earth’s surface?
Answers
It means that the air near the Earth's surface is denser compared to the other parts.
Air Density99.9% of the air particles are found in the lower part of the atmosphere because the air in this region is denser than the other regions.
Air density increases with increased pressure and as we move up in altitude, air pressure decreases.
Thus, as air pressure decreases with height, the density of air also decreases. This is why most of the air particles are found in the lower part of the atmosphere.
More on air density can be found here: https://brainly.com/question/23294784?referrer=searchResults
During a lab experiment performed at STP conditions, you prepare HCl by reacting 100. ml of Cl2 gas with an excess of H2 gas.
How many ml of a solution of Ba(OH)2 0.230M do you need to neutralize all the HCl produced?
Answers
Answer: 19.4 mL Ba(OH)2
Explanation:
H2(g) + Cl2(g) --> 2HCl(aq) (make sure this equation is balanced first)
At STP, 1 mol gas = 22.4 L gas. Use this conversion factor to convert the 100. mL of Cl2 to moles.
0.100 L Cl2 • (1 mol / 22.4 L) = 0.00446 mol Cl2
Use the mole ratio of 2 mol HCl for every 1 mol Cl2 to find moles of HCl produced.
0.00446 mol Cl2 • (2 mol HCl / 1 mol Cl2) = 0.00892 mol HCl
HCl is a strong acid and Ba(OH)2 is a strong base so both will completely ionize to release H+ and OH- respectively. You need 0.00892 mol OH- to neutralize all of the HCl. Note that one mole of Ba(OH)2 contains 2 moles of OH-.
0.00892 mol OH- • (1 mol Ba(OH)2 / 2 mol OH-) • (1 L Ba(OH)2 / 0.230 M Ba(OH)2) = 0.0194 L = 19.4 mL Ba(OH)2
Under what conditions will deviations from the "ideal" gas be expected?
Answers
Answer:
Conditions which results in deviating a gas from "ideal" behavior are
1. Low Temperature
2. High Temperature
Explanation:
Ideal gas according to the kinetic model theory states that the conditions that apply are high temperatures where kinetic energy and low pressure is too high and the interactions in between and the container are negligible. Hence, the deviations of ideal gas falls when there is low temperature and high pressure.
Which best describes the velocity of a person on a merry-go-round? * 1 point zero constant increasing continuously changing

Answers
Answer:
continuously changing
Explanation:
The velocity of a person on a merry-go-round can best be described as continuously changing. This is because the initial velocity before the ride starts would be 0. Once the ride begins, it keeps going faster and faster until it reaches a certain speed. During this time the velocity is increasing. Then it remains constant for a certain period of time and when the ride is over the velocity and speed begins to decrease until ultimately reaching a speed of 0. Therefore, it is continuously changing throughout the duration of the ride.
in a laboratory experiment, a student found that a 195-ml aqueous solution containing 2.494 g of a compound had an osmotic pressure of 12.2 mmhg at 298 k. the compound was also found to be nonvolatile and a nonelectrolyte. what is the molar mass of this compound?
Answers
The compound's mole ratio in a laboratory setup is 3125.12 g/mol, according to the statement.
Water: an electrolyte or not?Water that is completely free of ions is said to be pure. This could conduct electricity as a result. The inclusion of ions in the mixture allows the solution to carry an electric current when other substances, such as ionic compounds, are dissolved in water.
Briefing:Osmotic pressure is given by;
π = C * R * T
∵ 760 mmHg = 1 atm
10.3 mmHg = 1 * 12.2/760 atm
= 0.016 atm
π = no. of moles * R * T/volume (L)
π = Weight (g) * R * T / Molar mass * V (L)
0.016 atm = 2.494 * 0.082 L atm K⁻¹ mol⁻¹ * 298 K/ M.W * 195 * 10⁻³
M.W = 3125.12 g/mol
Consequently, the compound's molar mass is 3125.12 g/mol.
To know more about Nonelectrolyte visit:
https://brainly.com/question/29771118
#SPJ1
all atoms in an element have the same number of protons. T/F
Answers
Yes, atoms of the same element contain the same number of protons.
The reason for this is that the number of protons in the nucleus is the atomic number of the element. Due to the uniqueness of the atomic number, it is considered a way to identify elements For example, hydrogen has one proton in its nucleus.
Do all the atoms of an element have the same number of protons?
The number of protons in an atom is called its atomic number (Z). This number is very important because it is unique for atoms of a given element. All atoms of an element have the same number of protons, and every element has a different number of protons in its atoms.
Are all atoms the same in an element?
No. Two atoms of the same chemical element are typically not identical. First of all, there is a range of possible states that the electrons of an atom can occupy. Two atoms of the same element can be different if their electrons are in different states
How many protons are in every atom?
The number of protons in an atom is equal to the atomic number of the element. For example, let's use oxygen. According to the periodic table, oxygen has the atomic number eight.
To know more about isotopes:
https://brainly.com/question/24720481
#SPJ4
Two balloons, one containing 1.54 L hydrogen gas and the other containing 0.72 L of
helium gas, are at the same pressure and temperature conditions. If the second balloon
contains 0.100 mol of helium, then the mass of hydrogen gas in the first balloon is:
A. 0.43 g
B. 0.22 g
C. 0.094 g
D. 0.047 g

Answers
Answer:
A.) 0.43 g
Explanation:
Before you can calculate the mass, you need to find the moles. You can do this by using Avogadro's Law:
V₁ / n₁ = V₂ / n₂
In this equation, "V₁" and "n₁" represent the first balloon's volume and mole value. "V₂" and "n₂" represent the second balloon's volume and mole value. Since you are searching for the first balloon's mole value, you can plug the other values into the equation and simplify to find n₁.
V₁ = 1.54 L V₂ = 0.72 L
n₁ = ? moles n₂ = 0.100 moles
V₁ / n₁ = V₂ / n₂ <----- Avogadro's Law
1.54 L / n₁ = 0.72 L / 0.100 moles <----- Insert values
1.54 L / n₁ = 7.2 <----- Simplify right side
1.54 L = 7.2 x n₁ <----- Multiply both sides by n₁
0.214 = n₁ <----- Divide both sides by 7.2
Now, you can find the mass using the molar mass of the gas. Remember, hydrogen exists as a diatomic molecule.
Atomic Mass (H₂): 2(1.008 g/mol)
Molar Mass (H₂): 2.016 g/mol
0.214 moles He 2.016 g
-------------------------- x ----------------- = 0.43 g H₂
1 mole
The label CORROSIVE on a chemical container indicates
Answers
The label CORROSIVE on a chemical container indicates that the contents are corrosive, meaning that contact with these materials can cause damage to living tissue and materials.
It is important to exercise caution when handling these materials and to ensure that the containers are well labeled and stored in a safe and secure location.
Corrosive chemicals are substances that can cause damage or destruction to other substances with which they come into contact by means of a chemical reaction. These reactions can occur on contact, or over a period of time. Corrosive chemicals can cause damage to the skin, eyes, respiratory tract, and other parts of the body.
Learn more about chemical container
https://brainly.com/question/29488607
#SPJ4
A student electrolysed some water. She collected 20 cm of oxygen, What volume of hydrogen could she also
have collected at the same time?
Answers
According to the chemical formula of water the volume of hydrogen collected on electrolysis of water will be double of water thus volume of hydrogen is 40 cm.
What is chemical formula?Chemical formula is a way of representing the number of atoms present in a compound or molecule.It is written with the help of symbols of elements. It also makes use of brackets and subscripts.
Subscripts are used to denote number of atoms of each element and brackets indicate presence of group of atoms. Chemical formula does not contain words. Chemical formula in the simplest form is called empirical formula.
Learn more about chemical formula,here:
https://brainly.com/question/29031056
#SPJ1
What is the pH range for an acid?
0-7
7-14
7
0
Answers
Explanation:
If the pH is between 0 and 7, then we have an acid.
If the pH is between 7 and 14, then we have an alkaline base.
If pH = 7, then it's neutral.
Answer:
0-7
Explanation:
Any substance that has a pH of less than 7 is acidic - 0 is a strong acid whereas 6 is a weak acid. A pH of 7 means that the substance is neutral. Anything above pH 7 is alkaline, pH 8 is a weak alkaline and pH 14 is a strong alkaline.Hope this helps!
why helium is inactive
Answers
why helium is inactive
Answer:- Outer shell of the He (1s) is completely filled with the 2 electrons He has very low intermolecular forces so it donot get liquefied even Due to complete duplet it has stable configuration so it doesn't required to react with another atoms to attain stabilityDue to high stability it is also placed among noble gases which are also called inert gasesPlease help me on this

Answers
..
For each of the following gas-phase reactions, indicate how the rate of disappearance of each reactant is related to the rate of appearance of each product: (c) N21g2 + 3 H21g2¡2 NH31g2
Answers
The following gas-phase reactions, the rate of disappearance of each reactant and the rate of appearance of each product for the reaction: N2(g) + 3H2(g) → 2NH3(g) is related to each other by a stoichiometric factor.
For the given gas-phase reaction: N2(g) + 3H2(g) → 2NH3(g), the stoichiometric coefficients of the reactants and the product are 1, 3, and 2 respectively. The balanced chemical equation indicates that one mole of N2 reacts with three moles of H2 to produce two moles of NH3. Therefore, the rate of disappearance of N2 is related to the rate of appearance of NH3 by a factor of 1/2. That is, for every 1 mole of N2 that disappears, 2 moles of NH3 will appear.
Similarly, the rate of disappearance of H2 is related to the rate of appearance of NH3 by a factor of 3/2. That is, for every 3 moles of H2 that disappears, 2 moles of NH3 will appear. Therefore, the rate of disappearance of each reactant and the rate of appearance of each product for the given reaction are related to each other by a stoichiometric factor.
Learn more about stoichiometric coefficients at:
https://brainly.com/question/12733510
#SPJ11
Another alloy called nichrome contains only the elements chromium and nickel how much nickel dies it have
Answers
Answer:
80 g
Explanation:
Alloy = nichrome
100 g of nichrome contains 20 g of chromium
Contains only two elements (Nickel and Chromium) hence;
Mass of Alloy = Mass of Nickel + Mass of Chromium
100 = Nickel + 20g
Nickel = 100 g - 20 g = 80 g
The Independent Variable is:
Kinetic Energy
Height
Mass
Speed
Answers
The following reaction is what type of chemical reaction?
Na + MgCl → NaCl + Mg
A. single replacement
B. combustion
C. Combination
D. double replacement
Answers
Answer:
A. Single replacement
Explanation:
Na replaces Mg as the cation
An engineering team has a goal of developing a bicycle frame that is both
lighter and stronger than previous models. Which cost-benefit analysis has
left out potential risks?
A.increased sales compared to employee stress and loss of natural resources
B. Better function compared to ecological damage of mining materials and research a development time
C. Better function compared to uncertain supply of materials and research a development time
D. Increased sales compared to research and development time and cost of materials
Answers
Answer:
D
Explanation:
D
Answer:D. Increased sales compared to research and development time and cost of materials
Explanation:
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
I NEED HELP ASAP! I don’t know like how to draw it
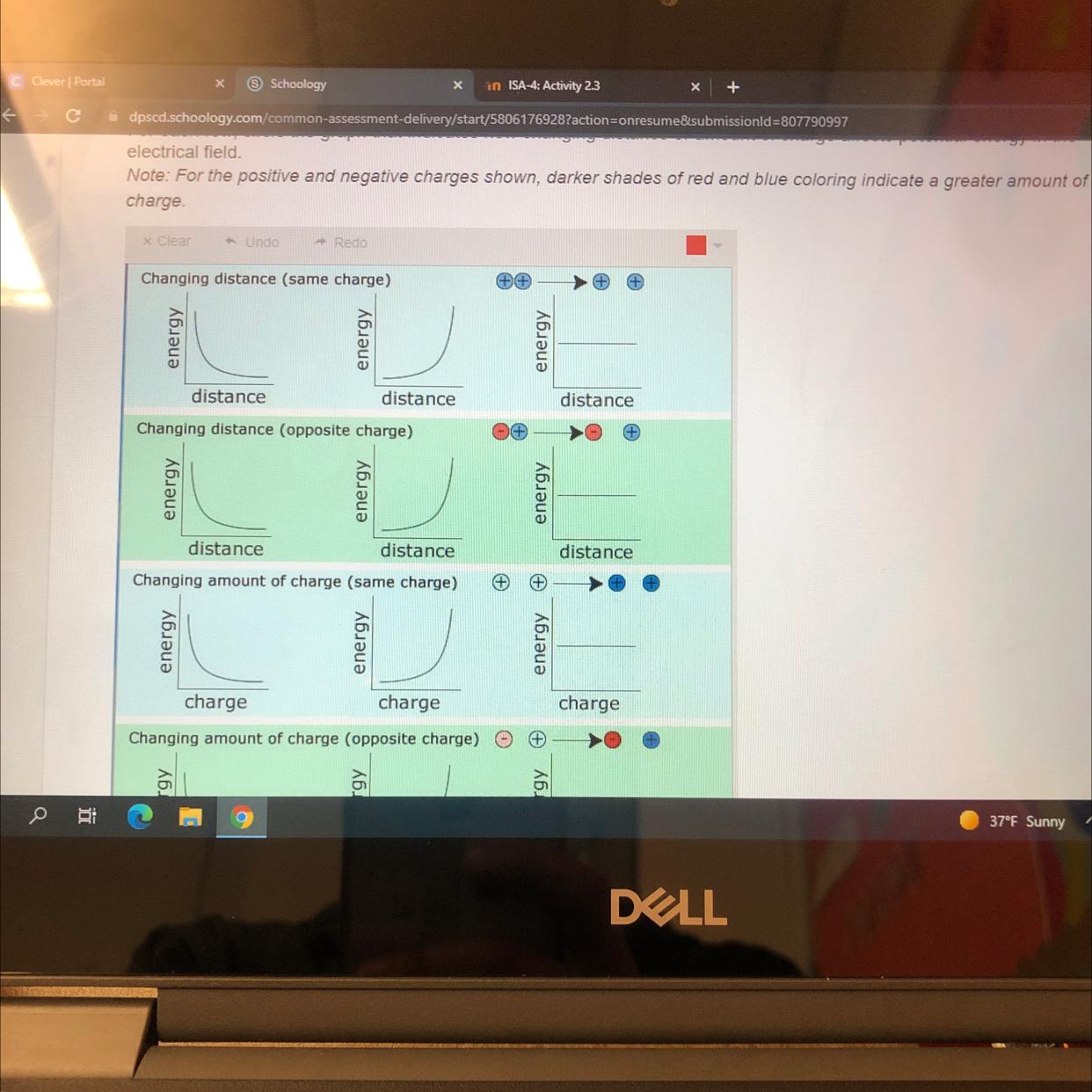
Answers
Explanation:
Take the first 6 going down and that's how your gonna do it
how many milliliters of 0.090 m naoh are required to titrated 50.0 ml of 0.090 m benzoic acid (hc7h5o2) to the equivalence point? use 3 significant figures for your answer.
Answers
50 mL of 0.090 M NaOH is required to titrate 50.0 mL of 0.090 M benzoic acid to the equivalence point.
We need to use the balanced chemical equation for reaction:
\(HC_7H_5O_2 + NaOH\ - > NaC_7H_5O_2 + H_2O\)
From the equation, we can see that the molar ratio of benzoic acid to NaOH is 1:1.
Therefore, the number of moles of NaOH required to reach the equivalence point is equal to the number of moles of benzoic acid:
moles of NaOH = moles of benzoic acid = \((0.090 M)(0.050 L)\)
= 0.0045 moles
To calculate the volume of 0.090 M NaOH needed to reach the equivalence point, we can use the molarity and moles of NaOH:
The volume of NaOH = moles of NaOH / molarity of NaOH
The volume of NaOH = \(0.0045 moles / 0.090 M = 0.050 L\)
(50 mL).
To know more about benzoic acid, here
brainly.com/question/24052816
#SPJ4
IWhich of the following solutions would be most likely to have the highest water concentration?
Multiple Choice hypertonic solution isotonic solution hypotonic solution water concentration and tonicity of a solution cannot be compared
Answers
"Hypotonic solution," refers to a solution with the highest water concentration due to its lower solute concentration compared to the other options, leading to water influx into cells.
A hypotonic solution would be most likely to have the highest water concentration. In a hypotonic solution, the solute concentration is lower than that inside the cell or compared to another solution. As a result, water tends to move into the cell or the solution to equalize the concentration.
When a cell is placed in a hypotonic solution, water molecules will move into the cell through the process of osmosis. This influx of water increases the water concentration inside the cell, leading to cell swelling or even bursting in extreme cases.
Compared to hypertonic and isotonic solutions, a hypotonic solution has a lower solute concentration, allowing for a higher concentration of water molecules. This results in a higher water concentration in the solution. It's important to note that the concept of tonicity is related to the relative solute concentrations between two solutions and their effect on cell osmosis. In this case, a hypotonic solution is characterized by a higher water concentration compared to the other options.
learn more about Hypotonic solution here:
https://brainly.com/question/28020628
#SPJ11
Perform the following
mathematical operation, and
report the answer to the
appropriate number of
significant figures.
1204.2 +4.79613 = [ ? ]

Answers
This problem is providing a mathematical expression which the result should be expressed with the correct significant figures. At the end, the result is 1209.0 because of the following:
Significant figures:In science, the use of significant figures is crucial as long numbers are not necessarily required when reporting a numerical value, for that reason the importance of reporting measurements with the correct number of significant figures.
In the case of additions, we perform the normal operation as the first step:
1204.2 +4.79613 = 1208.99613
Next, we round the result to the least number of decimal places, in this case one because 1204.2 has just one decimal place, unlike the 4.79613 which has five, so that we round the 8 up to 9 and leave a 0 as the only decimal place:
1209.0
Learn more about significant figures: https://brainly.com/question/11904364
The temperature of some air is minus 20 degrees C at 95kPa of pressure. What is the potential temperature, assuming a reference pressure at sea level (101.3kPa) ? Give your answer in degrees C, to the nearest degree.
Answers
The potential temperature is 15°C.
Given,The temperature of some air is minus 20 degrees C at 95 kPa of pressure.
Reference pressure at sea level = 101.3 kPa
The potential temperature (θ) is the temperature a parcel of dry air would have if it were adiabatically brought to a standard reference pressure, typically 1000 millibars (100 kPa).
Potential temperature is directly proportional to the absolute temperature and inversely proportional to the pressure in a system.
In order to find the potential temperature of the given air, we can use the formula below:
θ = T × (P0 / P)^(R/cp)
where,θ = potential temperature (in Kelvin)
T = temperature (in Kelvin)
P0 = reference pressure (in Pa)
P = actual pressure (in Pa)
R = gas constant for dry air (287 J/(kg·K))
cp = specific heat of dry air at constant pressure (1004 J/(kg·K))
Converting the given temperature in Celsius to Kelvin:
T = -20°C + 273.15K= 253.15K
The formula can be written as:
θ = T × (P0 / P)^(R/cp)θ
= 253.15 × (101300/95000)^(287/1004)θ
= 288.5 K
Converting the potential temperature from Kelvin to Celsius:
θ = 288.5 K - 273.15
= 15.35°C (to the nearest degree)'
= 15°C (rounded off to the nearest degree).
Therefore, the potential temperature is 15°C.
Learn more about the potential temperature from the given link-
https://brainly.com/question/4735135
#SPJ11
6. Which subatomic particle has no charge?
O proton
O electron
O neutron
Answers
Answer:
O Neutron
Explanation:
Answer:
the anwser is
electronHOPE IT HELPEDA hydrogen atom is excited from its ground state to the n= 4 state. The atom subsequently emits three photons, one of which has a wavelength of 1882 nm. What are the wavelengths of the other two photons?
Answers
The wavelength of the first line (n=4 to n=3) in the Balmer series is 656.3 nm. The wavelength of the second line (n=4 to n=2) in the Balmer series is 486.1 nm. The wavelength of the third photon, calculated above, is 594.1 nm.
The hydrogen atom has a single electron in its shell. Electrons in a hydrogen atom have a set of allowed energy levels. The ground state has the lowest energy level, and n represents the electron's energy level in the hydrogen atom.
Electrons gain energy and rise to a higher energy level when they are stimulated by energy from an external source such as an electric current or a photon beam.The hydrogen atom is said to be excited when its electron absorbs energy and rises to a higher energy level.
The excited atom then emits the energy in the form of electromagnetic radiation, which includes visible light and other wavelengths.The hydrogen atom has a characteristic spectrum due to the transitions between its energy levels. The wavelengths of photons absorbed or emitted when electrons in a hydrogen atom move between energy levels are determined by the following equation:
E=(hc)/λ whereE = energy of the photonh = Planck's constanctc = speed of lightλ = wavelength of the photonLet us now determine the wavelengths of the other two photons. When a hydrogen atom is excited from its ground state to the n=4 state, it emits a series of spectral lines known as the Balmer series.
The Balmer series is a series of lines in the visible light region of the spectrum. The wavelength of the first line (n=4 to n=3) in the Balmer series is 656.3 nm. The wavelength of the second line (n=4 to n=2) in the Balmer series is 486.1 nm.The third photon's wavelength can be calculated as follows
:Energy of the photon = (hc)/λEnergy of the photon = (6.626 x 10^-34 J s) x (2.998 x 10^8 m/s) / (1882 x 10^-9 m) = 3.328 x 10^-19 JThe wavelength of the third photon is calculated as follows: Energy of the photon = (hc)/λ3.328 x 10^-19 J = (6.626 x 10^-34 J s) x (2.998 x 10^8 m/s) / λλ = (6.626 x 10^-34 J s) x (2.998 x 10^8 m/s) / (3.328 x 10^-19 J)λ = 594.1 nmThe first two wavelengths are given by the Balmer series.
To know more about wavelength visit:
https://brainly.com/question/31143857
#SPJ11
ninety-six percent of the body mass is made from? a. calcium, phosphorus, oxygen, and nitrogen
b. carbon, hydrogen, carbon dioxide, and nitrogen
c. carbon, calcium, hydrogen, and nitrogen
d. carbon, oxygen, hydrogen, and nitrogen
Answers
The correct answer is option D. Ninety-six percent of the body mass is made up of carbon, oxygen, hydrogen, and nitrogen. These four elements are fundamental building blocks of organic compounds found in living organisms.
They form the basis of carbohydrates, lipids, proteins, and nucleic acids, which are essential components of cells and tissues in the human body. The combination of carbon, oxygen, hydrogen, and nitrogen enables the formation of complex molecules necessary for various biological processes and functions. Carbon, oxygen, hydrogen, and nitrogen are vital elements that constitute a significant portion of the human body's mass. Carbon is the backbone of organic molecules due to its unique ability to form stable covalent bonds with other atoms, including itself. It is present in carbohydrates, lipids, proteins, and nucleic acids, which are major components of cells and tissues. Oxygen is essential for cellular respiration, the process by which cells convert nutrients into energy. It is a crucial component of water (H2O) and participates in many biochemical reactions. Hydrogen, the lightest and most abundant element, is found in water and various organic compounds. It plays a role in maintaining pH balance, serving as a transport molecule, and participating in chemical reactions within cells. Nitrogen is a key component of proteins and nucleic acids, including DNA and RNA. Proteins are involved in various structural, enzymatic, and regulatory functions in the body, while nucleic acids carry genetic information and participate in protein synthesis. Together, carbon, oxygen, hydrogen, and nitrogen make up approximately 96% of the body's mass, highlighting their crucial roles in the composition and functioning of living organisms.
Learn more about nucleic acids here: brainly.com/question/11737667
#SPJ11
Will these magnets be attracted or repelled?
Answers
Explanation:
Magnets actually do both attract and repealto each other. Magnets repeal similar poles where as attract opposite poles.
What are ways in which minerals are used for items typically found in a medicine cabinet?
Answers
Ways in which minerals are used for items typically found in a medicine cabinet are: Emery boards have either garnet or corundum, Fluoride in toothpaste is derived from fluorite, and “Scouring agents” in toothpaste contain calcite.
Things in your domestic that are made with metallic mineral assets are simple to recognize. They are commonly glossy and see like metal unless they are painted, are difficult unless they are exceptionally lean, and are by and large overwhelming for their size. There are numerous other things in your domestic that are made of non-metallic mineral assets, too called mechanical minerals. The concrete establishment of your domestic is made with sand, rock, and lime from limestone. Chimney bricks are made of clay minerals. Window glass is made of quartz sand, whereas interior dividers are made of sheets of drywall made generally of the mineral gypsum. Gypsum is additionally ground up and utilized as a filler in paint on the dividers, and other minerals are ground up to supply shades that color the paint. All mineral assets are non-renewable, so it’s exceptionally critical that they are preserved. The washroom is made utilizing so numerous mineral assets that it is essentially a mine in disguise.
To know more about minerals refer to the link https://brainly.com/question/1333886?referrer=searchResults.
#SPJ4
Fumarase catalyzes the conversion of fumarate to L-malate. It has a Km of 2.0 mM for fumarate, and a Vm of 2.6 mmol-min-1 per mg enzyme. What would be the rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms
Answers
The rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms is approximately 0.975 mmol-min-1 per mg enzyme.
The rate of the reaction can be calculated using the Michaelis-Menten equation:
\(V = (Vm * [S]) / (Km + [S])\)
Where:
V is the rate of the reaction
Vm is the maximum velocity (2.6 mmol-min-1 per mg enzyme)
[S] is the substrate concentration (1.2 mM)
Km is the Michaelis constant (2.0 mM)
By substituting the values into the equation, we can find the rate of the reaction:
V = (2.6 mmol-min-1 per mg enzyme * 1.2 mM) / (2.0 mM + 1.2 mM)
V = (3.12 mmol-min-1 per mg enzyme) / (3.2 mM)
V ≈ 0.975 mmol-min-1 per mg enzyme
Therefore, the rate of the reaction when the fumarate concentration is 1.2 mM and the amount of enzyme is 100 micrograms is approximately 0.975 mmol-min-1 per mg enzyme.
To know more about rate of the reaction, refer
https://brainly.com/question/24795637
#SPJ11