Answers
Answer:
-226.0kJ = ΔH°f COCl₂(g)
Explanation:
Using Hess' law, it is possible to obtain the enthalpy of formation of a substance from the enthalpy change of a reaction and the other enthalpies of formation involved in the reaction.
For the reaction:
CO(g) + Cl₂(g) → COCl₂(g)
Hess's law is:
ΔHr = -115.5kJ = ΔH°f COCl₂(g) - (ΔH°f CO(g) + ΔH°f Cl₂(g))
ΔH°f CO(g) is -110.5kJ/mol
ΔH°f Cl₂(g) is 0 kJ/mol
Replacing in Hess's law:
-115.5kJ = ΔH°f COCl₂(g) - (-110.5kJ/mol + 0kJ/mol)
-115.5kJ = ΔH°f COCl₂(g) + 110.5kJ
-226.0kJ = ΔH°f COCl₂(g)Related Questions
he decomposition of NaClo3 is a first order reaction. A sample of NaClo3 was 90% decomposed in 48 minutes. How long would it take for a sample NaclO3 to be 50% decomposed
Answers
The time taken for 50% to be decomposed is the half life and this is 14.4 mins.
What is the time taken?We know that a first order reaction would have a half life that can be obtained from the formula;
N/No = (1/2)^ t/\(t_{\frac{1}{2} }\)
N = Number of atoms at time t
No = Number of atoms initially present
t = time taken
\(t_{\frac{1}{2} }\) = Half life of the reaction
Thus;
We have that the substance is 90% decomposed so only 10% of the original sample remains.
Now;
0.1No/No = (1/2)^48/\(t_{\frac{1}{2} }\)
0.1 = (0.5)^48/\(t_{\frac{1}{2} }\)
ln (0.1) = 48/\(t_{\frac{1}{2} }\) ln(0.5)
-2.3 = 48/\(t_{\frac{1}{2} }\) (-0.69)
48/\(t_{\frac{1}{2} }\) =-2.3/-0.69
\(t_{\frac{1}{2} }\) = 14.4 min
Learn more about half life:https://brainly.com/question/24710827
#SPJ1
what is the full answer with steps
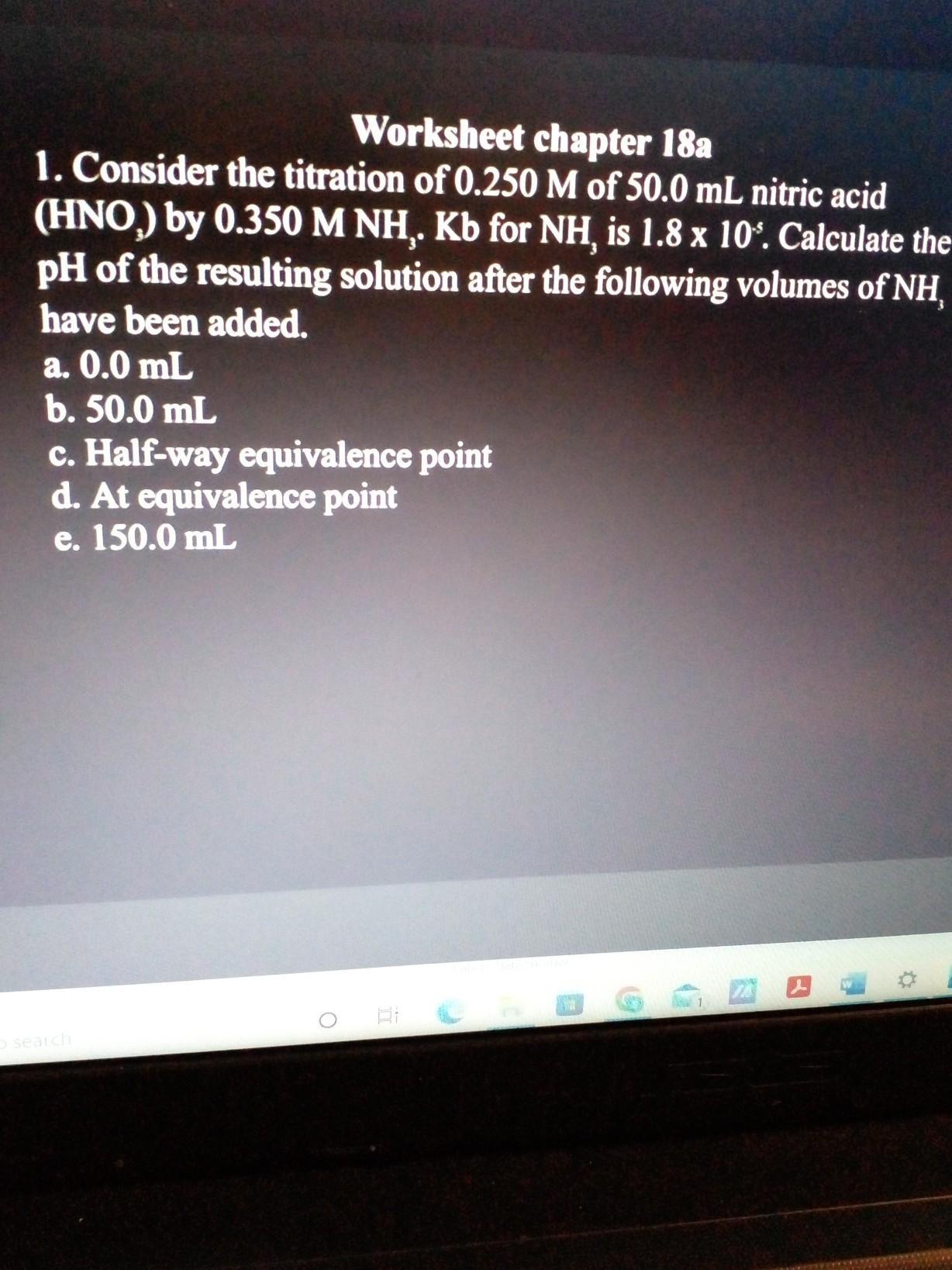
Answers
After adding the next amounts of NH, the final solution's pH was 4.98. Strong acid plus weak base equals an acidic pH, which is supported by this.
If you'll note, NH3 and HNO3 have the same volume and concentration, while HNO3 only contains one H.
As a result, you should persuade yourself that these react exactly as they should because their mol quantities are equivalent.
Make use of the following equation to determine pH:
pH = -log [H+]
The pH formula can be used to determine the pH of a chemical solution and how acidic or basic it is: pH equals -log10[H 3 O +]. Anything below 7 is acidic, and everything over 7 is basic. Learn how to calculate the pH of any chemical solution using the procedures listed below.
To Identify pH true meaning:
The concentration of hydrogen ions in a solution is determined by the pH scale. A solution having a lot of hydrogen ions in it is acidic. Normally, the pH scale is displayed from 0 to 14. The solution is more straightforward the larger the number.
To learn more about Calculating pH, visit
https://brainly.com/question/13008689
#SPJ13
A gas has a volume of 550 mL at a temperature of -55 °C. The volume of the gas at 30 °C is
Blank 1:
mL.
Answers
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
The volume of the gas at 30 °C is approximately 760.67 mL.
To determine the volume of the gas at 30 °C, we can use the combined gas law equation, which relates the initial and final conditions of temperature and volume for a gas.
The combined gas law equation is:
(P1 * V1) / (T1) = (P2 * V2) / (T2)
Where:
P1 and P2 are the initial and final pressures, respectively
V1 and V2 are the initial and final volumes, respectively
T1 and T2 are the initial and final temperatures in Kelvin, respectively
We need to convert the temperatures from Celsius to Kelvin by adding 273.15 to each value.
Given:
V1 = 550 mL
T1 = -55 °C = 218.15 K
T2 = 30 °C = 303.15 K
Assuming the pressure remains constant, we can rearrange the equation to solve for V2:
V2 = (P1 * V1 * T2) / (P2 * T1)
Since the pressure is not specified in the problem, we can assume it remains constant, allowing us to cancel out the pressure terms. Thus, the final equation becomes:
V2 = (V1 * T2) / T1
Plugging in the given values:
V2 = (550 mL * 303.15 K) / 218.15 K
Simplifying the calculation, we find:
V2 ≈ 760.67 mL
Therefore, the volume of the gas at 30 °C is approximately 760.67 mL.
For more question on gas law
https://brainly.com/question/27870704
#SPJ8
If your equation includes 7(CrO4)2, how many Cr's are there?
If your equation includes 7(CrO4)2, how many O's are there?
Answers
If an equation includes 7(CrO₄)₂, the numbers of Cr's and O's atoms that are there are 14 and 56 respectively.
How to calculate number of atoms?The number of atoms present in a chemical compound can be calculated by multiplying the subscript of the particular element by any coefficient.
According to this question, 7 moles of chromate with the chemical formula; (CrO₄)₂ is given. The number of oxygen and chromium atoms in this compound can be calculated as follows:
Chromium = 7 (coefficient) × 2 = 14 atomsOxygen = 7 (coefficient) × 8 = 56 atomsLearn more about no of atoms at: https://brainly.com/question/14190064
#SPJ1
Do you agree that heat can do work by constructing a model? Explain
Answers
Work can be totally transformed into heat (for example, by friction), whereas heat can only be partially converted into work.
What is a model?A model is a depiction of something that is frequently too difficult to directly observe or display. Although experimental testing validates a model, it is only accurate in explaining specific characteristics of a physical system.
Work is defined as the transfer of energy to or from a system by any means other than heat.
A transfer of energy to or from a system by any means other than heat is called “work”. Work can be completely converted into heat (by friction, for example), but heat can only be partially converted to work. The Second Law of Thermodynamics, states that the complete conversion of heat into work is impossible.
Heat transmission in materials can be described using the kinetic particle model. Heat energy always travels from a high-temperature zone to a low-temperature region.
Thus, heat can do work by constructing a model.
To learn more details about heat here:
brainly.com/question/1429452
#SPJ1
Which of the following shows the wavelengths of light that an atom gives off
when an electron falls to a lower energy level?
O A. Quantum spectrum
O B. Electromagnetic spectrum
O C. Emission spectrum
O D. Absorption spectrum
Answers
Emission spectrum shows the wavelengths of light that an atom gives off when an electron falls to a lower energy level. Hence, Option C is the correct answer.
What is Wavelength of ight ?The wavelength of light is defined as “The distance between the two successive crests or troughs of the light wave”.
Light is absorbed or emitted when an electron jumps or falls into an energy level.
The energy of light absorbed or emitted is equal to the difference between the energy of the orbits.
Therefore , the wavelengths of light that an atom gives off when an electron falls to a lower energy level corresponds to Emission spectrum.
Hence , Option C is the correct answer.
To know more about Emission Spectrum
brainly.com/question/13537021
#SPJ1
How many decay product atoms will be present in the rightmost sample, after two half-lives have passed?

Answers
Since Half-life has to do with the decay of half of the amount of an atom, after two half-lives, we will end up with 2 of quantity of Hg-207, since we have started with 8 particles, and after one half-life we will have 4, therefore the last one will have only 2 particles of Hg-207
What is the molarity of 30.0 mL of hydrochloric acid solution after 15.0 mL of a 3.00 M solution has been diluted?
___ M (Answer Format X.X)
Answers
Answer:
To find the molarity of the new solution, we need to use the equation:
M1V1 = M2V2
Where:
M1 = initial molarity = 3.00 M
V1 = initial volume = 15.0 mL
M2 = final molarity (what we're solving for)
V2 = final volume = 30.0 mL
Rearranging the equation to solve for M2:
M2 = (M1V1)/V2
M2 = (3.00 M * 15.0 mL)/30.0 mL
M2 = 1.50 M
Therefore, the molarity of the new solution is 1.50 M.
Answer:
1.00 M
Explanation:
Molarity is defined as the number of moles of solute per liter of solution. When a solution is diluted, the number of moles of solute remains constant, but the volume of the solution increases. Therefore, the molarity of the solution decreases.
In this case, the initial number of moles of solute in the 15.0 mL of 3.00 M hydrochloric acid solution is (15.0 mL) * (3.00 mol/L) * (1 L/1000 mL) = 0.045 mol.
After dilution, the volume of the solution increases to 30.0 mL + 15.0 mL = 45.0 mL. The molarity of the diluted solution is (0.045 mol) / (45.0 mL) * (1000 mL/L) = 1.00 M.
So, the molarity of 30.0 mL of hydrochloric acid solution after 15.0 mL of a 3.00 M solution has been diluted is 1.00 M.
A gas occupying 0.6 L at 1.70 atm expands to 0.9 L. What is the new pressure assuming temperature remains constant?
Answers
Answer:
1.13 atmExplanation:
The new pressure can be found by using the formula
P1V1 = P2V2
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
Since we're finding the new pressure P2 we make P2 the subject
We have
\(p_2 = \frac{p1v1}{v2} \\ \)
P1 = 1.7 atm
V1 = 0.6 L
V2 = 0.9 L
We have
\(p_2 = \frac{1.7 \times 0.6}{0.9} = 1.13333...\\ \)
We have the final answer as
1.13 atmHope this helps you
Which of these is not used to make models of interstellar medium?
Material from meteorites.
Minerals.
Synthetic materials.
Burnt wood ash.
Answers
Material from meteorites are not the elements used to make models of interstellar medium. The correct option is A.
What is interstellar medium?The matter and radiation that prevail in the space between star systems in a galaxy are referred to as the interstellar medium in astronomy. This matter consists of gas including ionic, atomic, and molecular, dust, and cosmic rays.
Meteorite material is not used to create models of the interstellar medium.
Thus, the correct option is A.
For more details regarding interstellar medium, visit:
https://brainly.com/question/12497791
#SPJ1
PLEASE HELP ASAP!!
Consider FIVE types of solids:
Ionic (NaCl)
Metallic (Ca)
Covalent Network (Quartz, SiO2)
Polar Molecular (sugar, C6H12O6)
Non-polar molecule
RECALL THE PHYSICAL PROPERTIES -> hardness, brittleness, the conductivity of electricity and heat, melting and boiling points, solubility in water, etc.
1. Design an experimental procedure to test these properties with the procedures below.
-> the ones I have so far
- ionic solids -> use NaCl and dissolve in water to test the solubility
- conductivity - by putting the solid under two free ends of the wire
-> solubility - using boiling water for all as ionic solids break into ions & conduct electricity
- brittleness - using a hammer or any other form of stress (if brittle, tends to break under stress)
- hardness - using a hydraulic press/Rockwell testing
- melting/boiling point - add heat to a sample after placing in a beaker or test tube to test
SOME OTHER THINGS WE CAN USE (but I'm unsure as to what we can use it for): a thermal camera
2. WRITE A HYPOTHESIS for ONE TYPE of solid with a brief explanation.
3. Design a Table of Observations for your experiments.
Answers
The tests that can be used to determine the kinds of solids that have been listed are shown below.
What are the solid types?Here are some tests that can be used to show that a solid is:
Ionic (NaCl):
Solubility test: NaCl is highly soluble in water, and a high degree of solubility can confirm the ionic nature of NaCl.
Conductivity test: In its molten or dissolved state, NaCl conducts electricity due to the presence of charged ions.
Metallic (Ca):
Conductivity test: Metals such as Ca conduct electricity due to the presence of free electrons in their crystal structure.
Ductility and malleability test: Metals are ductile and malleable, and can be easily deformed under pressure.
Covalent Network (Quartz, SiO2):
Hardness test: Covalent network solids such as quartz are extremely hard due to the strong covalent bonds between atoms.
Melting point test: Covalent network solids often have high melting and boiling points due to the strong intermolecular forces between atoms.
Polar Molecular (sugar, C6H12O6):
Solubility test: Polar molecules such as sugar are soluble in polar solvents such as water but insoluble in nonpolar solvents.
Melting and boiling point test: Polar molecular solids have lower melting and boiling points compared to ionic or covalent network solids due to weaker intermolecular forces.
Learn more about ionic solids:https://brainly.com/question/15241125
#SPJ1
Which event takes place first in the stages before the brith of a star
Answers
Answer:
nuclear fusion
Explanation:
gravity pulls gas and dust together a protostar forms as mass increases nuclear fusion begins under high pressure
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
The pKa of acetic acid is approximately 4.76. What is the pH of a 0.05 M acetic acid solution in water? Choose the answer closest to the one you calculate.
Answers
Answer:
3.46
Explanation:
Hello,
This question can be solved using Henderson-Hasselbalch equation.
The dissociation equation of acetic acid in water is
CH₃COOH + H₂O ⇄ CH₃COO⁻ + H₃O⁺
pH = pKa + log([conjugate base] / [weak acid])
Conjugate base = acetic acid
Weak acid = water
pKa = 4.76
pH = 4.76 + log([0.05]/[1])
pH = 4.76 + log(0.05)
pH = 4.76 + (-1.30)
pH = 3.46
From the above solution, the pH of the solution is 3.46
The magnet on the right is moved toward the suspended magnet on the left.
Which statement describes the interactions of the magnets in terms of their magnetic fields
Answers
When the magnet on the right is moved toward the suspended magnet on the left, a repulsive force between the two magnets causes the suspended magnet to swing to the left; option B
What are magnets?Magnets are materials which exhibit magnetism or produces a magnetic field.
Magnets attract other objects to themselves and these objects are said to be magnetic.
Magnetic objects include metals such as iron.
A magnet has two ends called poles. The poles of a magnet are the North and South pole.
Magnetic lines of forces begin at the North pole whereas magnetic lines of forces end in at the south Pole.
The law of magnetism states that like poles of a magnet repel each other while unlike poles attract.
Considering the given magnets;
the magnet on the right is moved toward the suspended magnet on the left causing the suspended magnet to move to the left means that the end of the magnets repel each other.
This implies that the ends are two similar ends of the magnets.
In conclusion, unlike ends of a magnet attract whereas unlike ends repel.
Learn more about magnets at: https://brainly.com/question/14997726
#SPJ1
Note that the complete question is given below:
The magnet on the right is moved toward the suspended magnet on the left.
Which statement describes the interactions of the magnets in terms of their magnetic fields?
A. A repulsive force between the two magnets causes the suspended magnet to swing to the left.
B. A repulsive force between the two magnets causes the suspended magnet to oscillate like a pendulum.
C. An attractive force between the two magnets causes the suspended magnet to swing to the right.
D. An attractive force between the two magnets cause the suspended magnet to oscillate like a pendulum.
Much smaller particles move very rapidly outside this center,
and these particles are called

Answers
Answer:
electron are the partials which float around the nucleus
Baking soda with hydrochloric acid combine and bubbles form. Chemical or physical change.
Answers
Answer:
I believe it is a chemical change.
What do melting, evaporation, and sublimation have in common?
Answers
Explanation:
Sublimation describes a solid turning directly into a gas. Melting, on the other hand, occurs when a solid turns into a liquid. Water can, under the right circumstances, sublimate, though it usually melts at temperatures above 0 degrees Celsius or 32 degrees Fahrenheit. Carbon dioxide (CO2), however, is very different. The conditions that determine whether CO2 melts or sublimates are both temperature and atmospheric pressure.
The size of the orbital is determined by the
The size of the orbital is determined by the blank quantum number, so the size of the orbital blank as this value increases. Therefore, an electron in a blank orbital is closer to the nucleus than is an electron in a blank orbital.
quantum number, so the size of the orbital
The size of the orbital is determined by the blank quantum number, so the size of the orbital blank as this value increases. Therefore, an electron in a blank orbital is closer to the nucleus than is an electron in a blank orbital.
as this value increases. Therefore, an electron in a
The size of the orbital is determined by the blank quantum number, so the size of the orbital blank as this value increases. Therefore, an electron in a blank orbital is closer to the nucleus than is an electron in a blank orbital.
orbital is closer to the nucleus than is an electron in a
The size of the orbital is determined by the blank quantum number, so the size of the orbital blank as this value increases. Therefore, an electron in a blank orbital is closer to the nucleus than is an electron in a blank orbital.
orbital.
orbital.
Answers
Answer: The size of the orbital is determined by the principal quantum number, so the size of the orbital increases as this value increases. Therefore, an electron in a (n-1) orbital is closer to the nucleus than is an electron in a 'n' orbital.
Explanation:
In an atom, the position and energy of an electron is described by a set of numbers and these sets are called quantum numbers.
There are four quantum numbers. These are as follows.
1). Principal quantum number - This is denoted by "n" and it determines the size and energy of shell in which electron is present. The value of "n" can be 1, 2, 3, and so on but it can never be equal to zero.
2). Azimuthal quantum number - This is denoted by "l" and it determines the shape of an orbital. For s, p, d and f-shell the values of "n" will be 0, 1, 2, 3. The value of l can vary from -n to +n.
3). Magnetic quantum number - This is denoted by "\(m_{l}\)." and it determines the orientation of an orbital. The value of ml can vary from -l to +l.
4). Spin quantum number -- This is denoted by "\(m_{s}\)" and it determines the spin of an electron. It is independent of the values of n, l and \(m_{l}\).
This means that the size of an orbital is determined by principal quantum number. Lower is the value of 'n' (principal quantum number) more closer will be an electron to the nucleus. Hence, more is the value of 'n' more will be the size of nucleus and vice-versa.
For example, an electron present in a 2s-orbital is closer to the nucleus as compared to the electron present in a 3s-orbital.
Thus, we can conclude that the size of the orbital is determined by the principal quantum number, so the size of the orbital increases as this value increases. Therefore, an electron in a (n-1) orbital is closer to the nucleus than is an electron in a 'n' orbital.
Not a timed or graded assignment. Please show calculation work. Question prompt : “what is the maximum mass of calcium chloride (CaCI2) that can form?” Quick answer = amazing review :)

Answers
The equation presented to us is not balanced. We must balance it first. The elements that are not balanced are chlorine and hydrogen. We have two atoms of those elements in the reactants, so we put the coefficient two in front of the HCl molecule to balance.
\(CaCO_3+2HCl\rightarrow CaCl_2+H_2O+CO_2\)Now, the reaction is balanced, since we have the same number of atoms of each element on each side of the reaction. Now we must calculate the limiting reagent since it will be based on this reagent that we will do the calculations to find the mass of CaCl2.
To find the limiting reactant we calculate the moles of each reactant using its molar mass.
Moles of CaCO3
\(\begin{gathered} MolCaCO_3=10.0gCaCO_3\times\frac{1molCaCO_3}{100.086gCaCO_3} \\ MolCaCO_3=0.100MolCaCO_3 \end{gathered}\)Moles of HCl
\(\begin{gathered} MolHCl=15.0gHCl\times\frac{1molHCl}{36.45gHCl} \\ MolHCl=0.412molHCl \end{gathered}\)By stoichiometry, we can calculate the ratio HCl to CaCO3. We need two moles of HCl for one mole of CaCO3 to react. So the ratio HCl to CaCO3 is equal to 2/1. That is the theoretical ratio. The current is found by dividing the moles of HCl by those of CaCO3.
The actual ratio HCl to CaCO3 is 0.412/0.1=4.1
The current ratio is higher than the theoretical one, which means we have enough HCl to react. This will be the excess reagent. So the limiting reactant will be CaCO3.
By stoichiometry, we have that 1 mol of CaCO3 produces 1 mol of CaCl2. So, the maximum moles produced will be the same moles of CaCO3 available. This is 0.100mol of CaCl2.
The mass of CaCl2 will be:
\(\begin{gathered} MassCaCl_2=0.100molCaCl_2\times\frac{110.978gCaCl_2}{1molCaCl_2} \\ MassCaCl_2=11.1gCaCl_2 \end{gathered}\)The maximum mass of calcium chloride (CaCI2) that can form is 11.1 g of CaCl2.
What is the Scientific notation for 0.10050
Answers
Answer:
1.005 x \(10^{1}\)
Explanation:
Move the decimal once to the right
Question 1(Multiple Choice Worth 50 points)
(01.06 LC)
Which technology removes carbon dioxide from the air by binding to it?
Answers-
O Pyrolization
O Direct-air capture
() Engineered molecules
O Carbon ground injection
Answers
Answer:
Direct-air capture
Explanation:
To understand the technology I'm about to explain, I'll model it to something familiar. As you know, the process of photosynthesis is where trees and plants use sunlight, water, and carbon dioxide to create energy for themselves, and release oxygen as a byproduct. Direct- air capture is exactly like this. Its pulls in air and then through a range of similiar chemical reactions, it extracts the carbon dioxide from it while returning the rest of the air to the surrounding environment. Except, the Direct-air capture does this at a much faster rate than nature does. The direct-air capture uses a ginormous fan that pulls air in which then passes over plastic surfaces that have potassium hydroxide solutions covered over them. The potassium hydroxide solution chemically binds with the carbon dioxide molecules, removing them from the air and trapping them in the liquid solution as a carbonate salt. The carbon dioxide is then contained where it is compressed and purified and then stored for later use.
Is cheese melting on a hamburger conduction, convection, or radiation?
Answers
Answer:
convection
Explanation:
Answer:
Conduction
Explanation:
What is conduction? It is that heat is transferred from one particle of matter to another. It involves contact
What if you measured 15cc of liquid elemental mercury, how much would that weigh in pounds
Answers
Answer:
0.447 pounds
Explanation:
The density of Mercury in grams/mL is 13.55 - I multiplied that by the volume and got approximately 203.25 grams, I then turned it into Kilograms and multiplied by 2.2 lbs / kg.
I a lab situation, add magnesium to HCl and perform a reaction.a. Write the valences equation b. If you start with .11 grams of Mg, how much hydrogen gas (L) should you produce? (this is at STP since you are using 22.4L/mole of gas)c. If your classroom conditions are 23 Celsius and 105.2Kpa, what would be theoretical volume of hydrogen gas at these conditions? d. If the students actually collected 145 mL of gas (.145L), what is the % yield for the experiment?
Answers
So,
The reaction that we're performing is the next one:
\(Mg+HCl\to MgCl_2+H_2\)We need to balance it first:
\(Mg+2HCl\to MgCl_2+H_2\)To balance, we verify that the number of atoms of each element in the reaction is the same before and after it occurs. As you can see, we have the same amount of atoms of each type before and after so the reaction is balanced now.
Now, we want to find the amount of Hydrogen gas (in liters) that we would obtain if we start with 0.11 grams of Mg. For this, we could follow the next steps:
Step 1. First, we should pass the 0.11 grams of Mg to moles of Mg. To do this, we divide by the molar mass of Mg:
\(n=\frac{mass}{molar\text{ }mass}=\frac{0.11gMg}{\frac{24.3gMg}{molMg}}=0.00452675molesMg\)Step 2. Apply the stoichiometry of the given reaction and then use the fact that 1 mole of any gas is equal to 22.4L of the gas:
\(0.00452675molesMg\cdot\frac{1molH_2}{1molMg}\cdot\frac{22.4LH_2}{1molH_2_{}}=0.1014LH_2\)Therefore, we produce 0.1014 L of H2.
c. If the conditions are 23 Celsius and 105.2Kpa, we could use the ideal gas formula to find the theoretical volume of hydrogen gas.
\(PV=nRT\)Remember that 23 Celcius = 296K and 105.2Kpa = 1,0382atm.
Now, if we replace:
\((1.0382atm)(V)=(0.00452675molH_2)(\frac{0.082atmL}{molK})(296K)\)Solving for V, we obtain that the theoretical volume is:
\(V=\frac{(0.00452675molH_2)(\frac{0.082atmL}{molK})(296K)}{1.0382\text{atm}}=0.1058L\)Which is approximately equal to the volume of the point b.
Imagine that you mix 25 g of water at 25 ºC with 25 g of water at 65 ºC. Predict the final temperature of the sample.
Answers
The final temperature of the mixture given that 25 g of water at 25 °C is mixed with 25 g of water at 65 °C, is 45 °C
How do i determine the final temperature of the mixture?The final temperature of the mixture can be obtained by calculating the equilibrium temperature of the mixture. This is shown below:
Mass of cold water (M) = 25 gTemperature of cold water (T) = 25 °CMass of warm water (Mᵥᵥ) = 25 gTemperature of warm water (Tᵥᵥ) = 65 °CEquilibrium temperature (Tₑ) =?Heat loss by warm water = Heat gain by cold water
MᵥᵥC(Tᵥᵥ - Tₑ) = MC(Tₑ - T)
Cancel out C
Mᵥᵥ(Tᵥᵥ - Tₑ) = M(Tₑ - T)
25× (65 - Tₑ) = 25 × (Tₑ - 25)
Cancel out 25
65 - Tₑ = Tₑ - 25
Collect like terms
65 + 25 = Tₑ + Tₑ
90 = 2Tₑ
Divide both side by 2
Tₑ = 90 / 2
Tₑ = 45 °C
Thus, we can conclude that the final temperature the mixture is 45 °C
Learn more about temperature:
https://brainly.com/question/14281142
#SPJ1
Venus's atmosphere, while primarily CO2, is also 3.5% nitrogen gas (i.e. mole fraction of 0.035). What is the partial pressure of nitrogen on Venus in kPa given that the total atmospheric pressure is 1334 psi?
Answers
The partial pressure of nitrogen on Venus is approximately 321.914 kPa.
To find the partial pressure of nitrogen on Venus, we need to calculate the partial pressure using the mole fraction of nitrogen and the total atmospheric pressure. First, we convert the total atmospheric pressure from psi to kilopascals (kPa) since the mole fraction is given in terms of kPa.
1 psi = 6.89476 kPa
Therefore, the total atmospheric pressure on Venus is:
1334 psi × 6.89476 kPa/psi = 9197.53 kPa
Next, we can calculate the partial pressure of nitrogen using the mole fraction. The mole fraction of nitrogen is given as 0.035, which means that nitrogen makes up 3.5% of the total moles of gas in the atmosphere.
The partial pressure of nitrogen is given by:
Partial pressure of nitrogen = Mole fraction of nitrogen × Total atmospheric pressure
Partial pressure of nitrogen = 0.035 × 9197.53 kPa
Partial pressure of nitrogen = 321.914 kPa
Therefore, the partial pressure of nitrogen on Venus is approximately 321.914 kPa.
It's important to note that the given atmospheric composition of Venus's atmosphere and the total atmospheric pressure are approximate values and can vary depending on specific conditions and measurements.
For more such question on partial pressure visit:
https://brainly.com/question/19813237
#SPJ8
Many ravines can be found in forested areas of the Cross Timbers ecoregion of Texas. What natural process most likely formed
these ravines?
OA. erosion by water during the rainy season
OB. erosion by wind during the dry season
OC. deposition by water during the rainy season
OD. deposition by wind during the dry season
Answers
Erosion by water during the rainy season can be a significant natural phenomenon. When it rains, water flows over the land, picking up soil particles and carrying them along with it.
What is water ?Water is a clear, colorless, odorless, and tasteless liquid that is essential for life. It is a chemical compound made up of two hydrogen atoms and one oxygen atom, with the chemical formula H2O. Water is the most abundant substance on Earth, covering approximately 71% of its surface.
Water is a unique substance because of its molecular structure, which allows it to exist in all three physical states - solid, liquid, and gas - at temperatures and pressures commonly found on Earth. It has a high heat capacity, meaning it can absorb and release large amounts of heat without changing temperature significantly. This property is important for regulating the Earth's climate and for maintaining the temperature of living organisms.
Water is essential for many biological processes, such as hydration, digestion, and metabolism. It also plays a crucial role in the Earth's water cycle, where it evaporates from bodies of water and plants, condenses into clouds, and falls back to the surface as precipitation.
To know more about Water visit :
https://brainly.com/question/28465561
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
A sample of sugar (C12H22O11) contains
1.505 × 1023 molecules of sugar. How many moles of sugar are present in the sample? Answer without doing any calculations.
Answers
Answer: 0.25 mol
Explanation:
Use the formula n=N/NA
n= number of mols
N = number of particles
Nᵃ = Avogadros constant = 6.02 x
So, n=
The 10 to the power of 23 cancels out and you are left with 1.505/6.02, which is approximately 1/4. This is the same as 0.25 mol.
Hope this helped :)